CAPSULO: new ND:YAG laser by Lumibird Medical
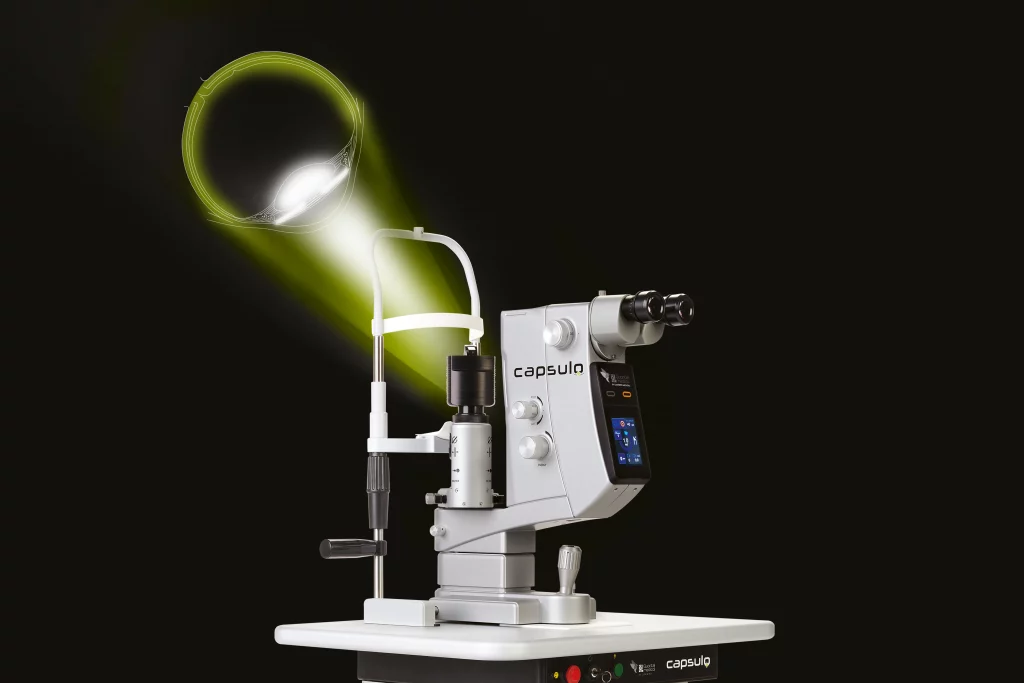
The value of Nd:YAG laser treatment for secondary cataract remains undisputed; and with the redesign of Quantel Medical’s flagship Nd:YAG Laser, Lumibird Medical delivers CapsuloTM; the next generation technology, continuing to support this important growing market need.
NEW WEBSITE: GLAUCOMA LASER ASSISTED SOLUTIONS

Lumibird Medical, leader in ophthalmic laser, today announces its new website: www.glaucoma-laser-assisted-solutions.com dedicated to laser therapies for each glaucoma stage.
C.STIM: New I.P.L System for the treatment of dry eye disease
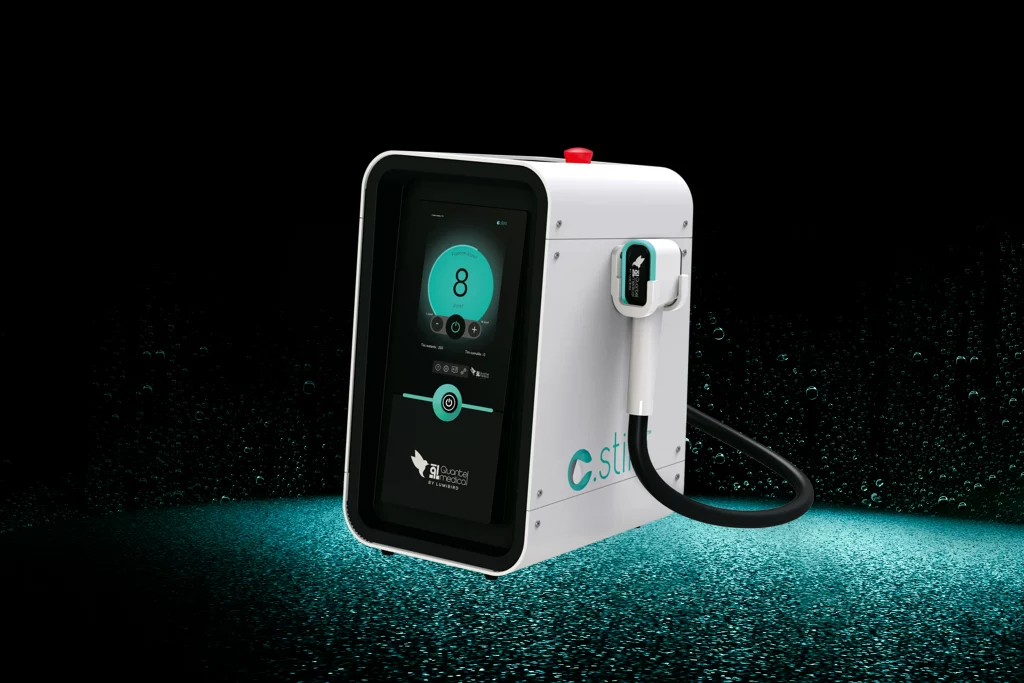
Quantel Medical today announced the launch of C.STIMTM IPL system. Based on intense pulsed light technology, C.STIMTM is the new generation of IPL designed by Quantel Medical to treat dry eye disease. Dry eye is a huge public health problem affecting more than 700 million people in the word and it is the most common pathology in ophthalmic visits….
NEW WEBSITE: SUBLIMINAL LASER THERAPY FOR RETINAL DISORDERS

Laser therapy has been used to treat retinal pathologies for decades. Over the years, the development and introduction of new and innovative laser technologies has confirmed that, alongside intravitreal injections, retinal laser therapy still plays a valuable role in the surgeons’ treatment armamentarium.
Lumibird Medical’s glaucoma treatment potential further strengthened by the latest EGS recommendations

The European Glaucoma Society (EGS), the world’s foremost learned society for glaucoma and its treatments, issued recommendations in its fifth “Terminology and Guidelines for Glaucoma” report, published in March 2021, that validate the positioning and relevance of the solutions developed by LUMIBIRD Medical. EGS officially added SLT…
Creation of 3 Lumibird Medical Nordics subsidiaries

The Lumibird Medical Group, the medical subsidiary of the Lumibird Group, the European leader in laser technologies, today announces the creation of Lumibird Medical Nordics AB in Sweden, Lumibird Medical Nordics OY in Finland and Lumibird Medical Nordics AS in Norway.
MOSAR® FOR EASYRET®: HIGH DEFINITION IMAGING SYSTEM
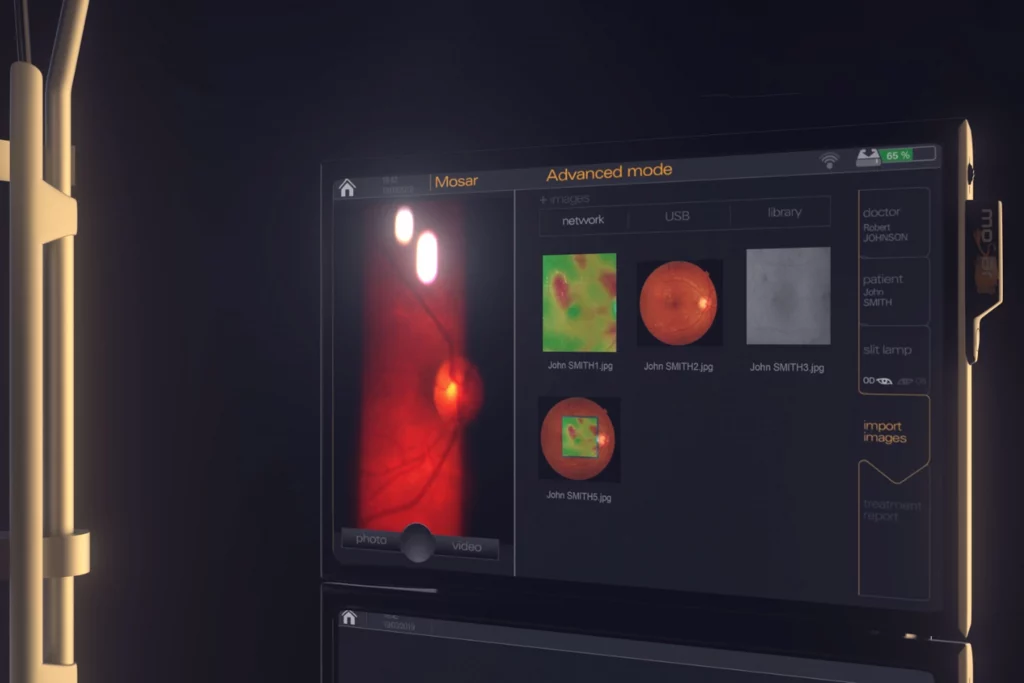
Quantel Medical accessorizes its yellow laser Easyret® with Mosar®, a High definition imaging system.
Always tuned to the market needs and to the feedbacks of the ophthalmologists, Quantel Medical launches Mosar®, a new optional imaging system dedicated to its high-end yellow retinal laser.
QUANTEL MEDICAL COMPLETES ACQUISITION OF ELLEX

Quantel Medical has completed its acquisition of Ellex, including the company’s laser and ultrasound technology solutions (with the exception of 2RT® and iTrack®), via the financial holding company Lumibird Medical.
Quantel Medical and Ellex announce merger agreement

Quantel Medical announces a major step forward for Quantel Medical and the Lumibird group as merger discussions have been initiated with Ellex, Adelaide, Australia.
VITRA 2 RECEIVES APPROVAL FROM THE FDA

Quantel Medical announced today that it has received approval from the U.S. Food and Drug Administration (FDA) for the Vitra 2® photocoagulator.