Quantel Medical and Ellex announce merger agreement

Quantel Medical announces a major step forward for Quantel Medical and the Lumibird group as merger discussions have been initiated with Ellex, Adelaide, Australia.
VITRA 2 RECEIVES APPROVAL FROM THE FDA

Quantel Medical announced today that it has received approval from the U.S. Food and Drug Administration (FDA) for the Vitra 2® photocoagulator.
Acquisition of the company Optotek Medical

Quantel Medical announces the acquisition of the Slovenian company Optotek Medical d.o.o., specialised in the development of optical solutions and lasers for medical applications.
LacryStim: CE mark approval
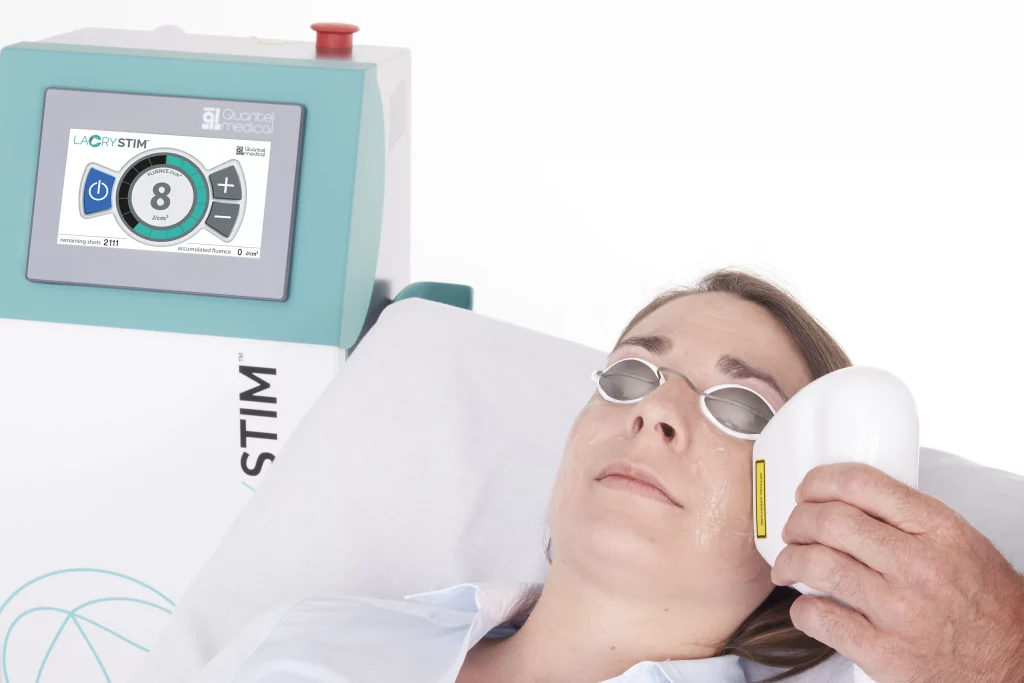
Dry eye disease is a multifactorial disease of the tears and ocular surface that can result in ocular discomfort and visual impairment. It affects all populations across the world with a major increase in people over 50 years old and is expected to increase as the population continues to age.
Quantel Medical receives FDA approval for the new A/B/S/UBM ultrasound platform ABSolu®
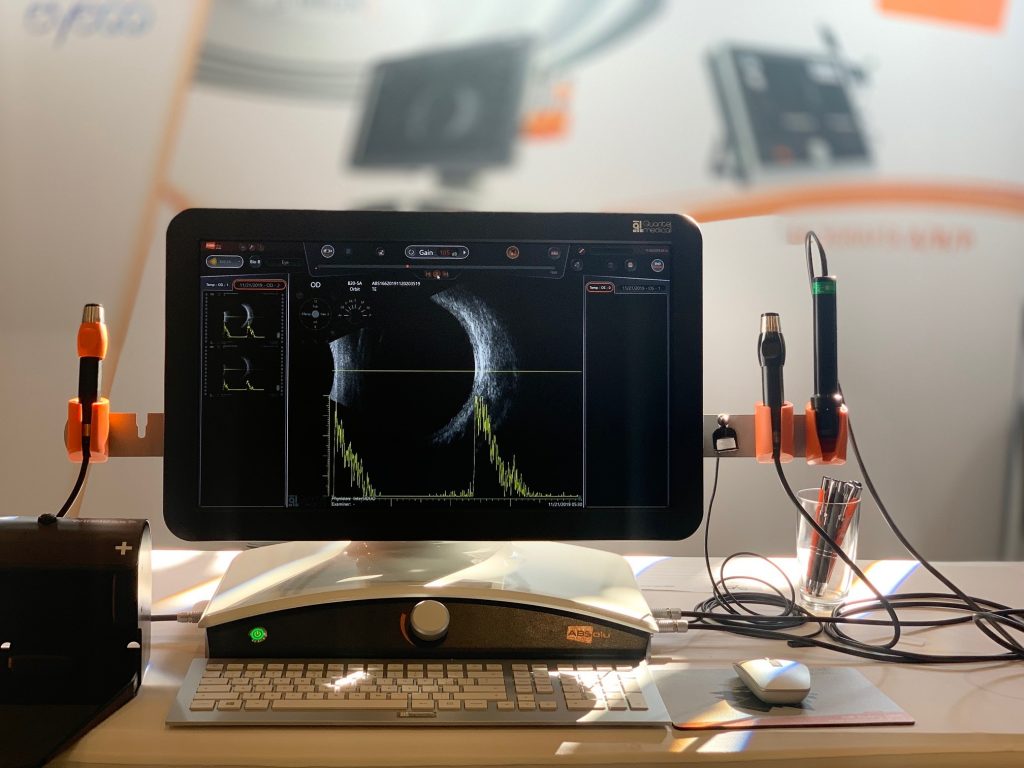
Quantel Medical today announced that it has received approval from the U.S. Food and Drug Administration (FDA) on March 25, 2019 for the New A/B/S Ultrasound Platform: ABSolu® .
Vitra 810 for Subcyclo: a new step forward in Subliminal Cyclophotocoagulation
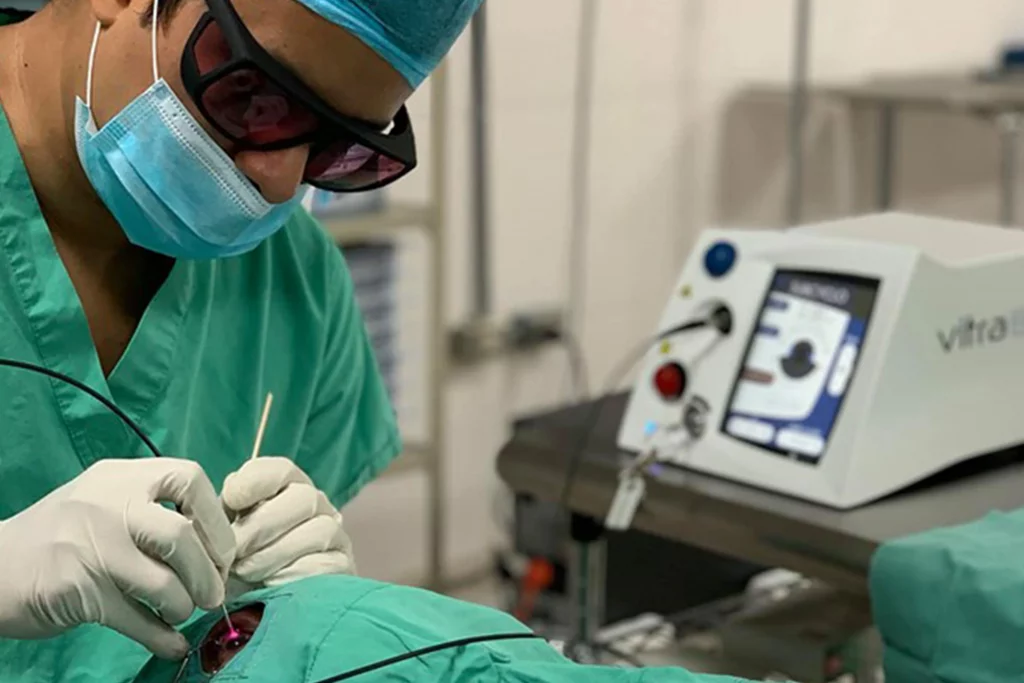
Quantel Medical, a global ophthalmic medical device company dedicated to developing leading technologies to improve the diagnosis and treatment of ocular diseases, today announced the launch of the new VITRA 810TM laser and its new SubCyclo® probe at the American Academy of Ophthalmology (AAO 2018) meeting being held in Chicago, USA, October 27…
ABSolu: new A/B/S/UBM ultrasound platform
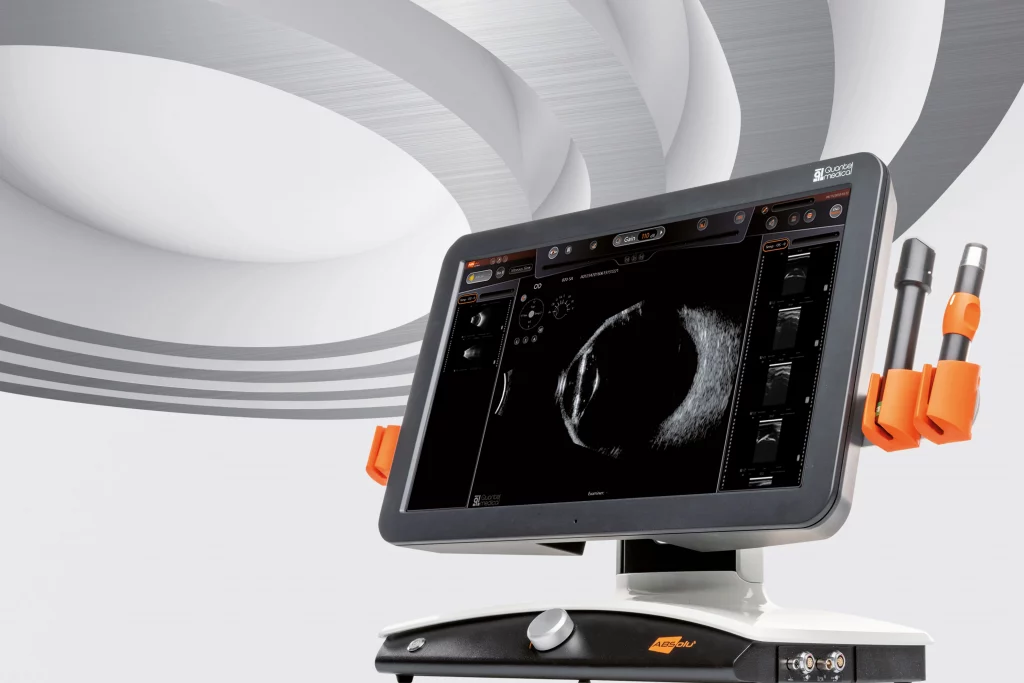
Quantel Medical today announced the launch of the new ABSoluTM ultrasound platform at the 36th Congress of the European Society of Cataract and Refractive Surgeons (ESCRS 2018) in Vienna, Austria September 22-26. Combination of leading edge technologies, this A/B/S/UBM ultrasound platform provides unparalleled image quality with an exceptional…
VITRA 2: Retinal Photocoagulation simplified

Quantel Medical reinvents its flagship 532nm green photocoagulator. Vitra 2 includes all the key elements that have made the success of the Vitra photocoagulator such as reliability, compactness and versatility.
Quantel Medical opens up new markets with its acquisition of ECM’s medical activities

Quantel Medical, a LUMIBIRD Group subsidiary, has taken a further step forward with its development by acquiring the medical activities of ECM, an IMV Technologies group subsidiary. World leader for ophthalmic diagnosis ultrasound solutions, Quantel Medical is opening up to other markets such as sports, general and hospital medicine.
Lacrydiag: complete diagnosis of the dry eye
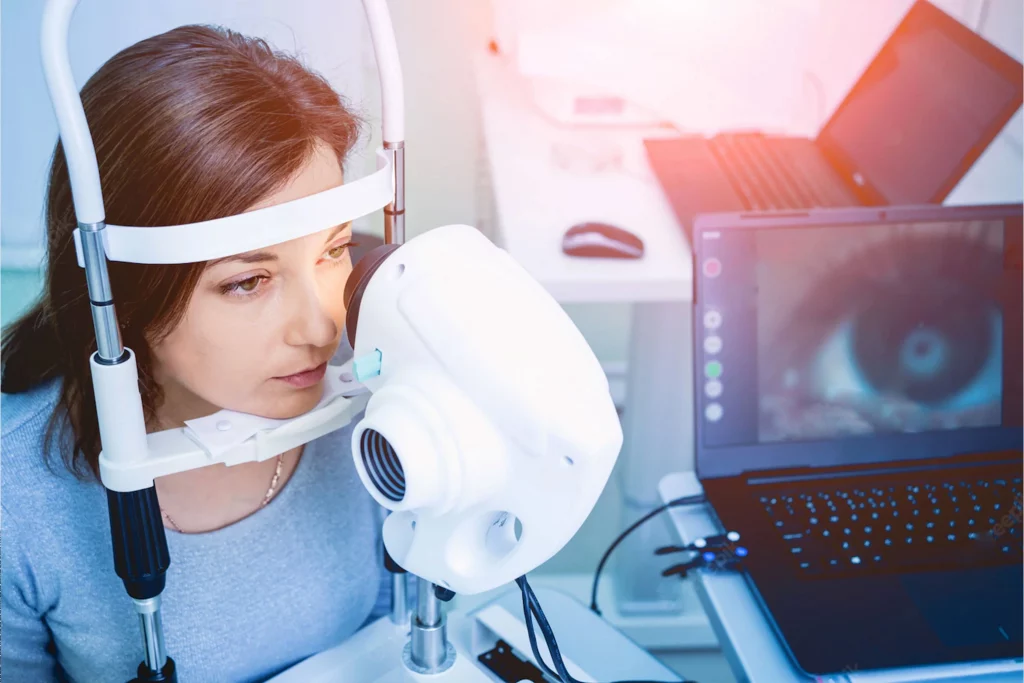
During the last World Ophthalmology Congress held in Barcelona, Spain, June 17-19, Quantel Medical launched LacryDiag, a complete diagnostic device for dry eye. LacryDiag is the first step of Quantel Medical in the dry eye market.